Microbial communities are the engines that drive the biosphere, playing critical biochemical functions in ecosystems and hosts, from soils to oceans to the human gut. The Gowda Lab’s guiding star is the pursuit of natural design principles underlying these functions. Nature “builds” community functions through the interplay of cellular physiology, community assembly, and evolutionary adaptation. This is pretty different from the ways that we as humans traditionally think about designing systems. Our view is that discovering these natural design principles via the integrated study of physiology, ecology, and evolution will teach us how to rationally manipulate and robustly engineer complex microbiomes for the benefit of human health.
The Gowda Lab is an interdisciplinary team of researchers in the Department of Microbiology at the Ohio State University who integrate experiments with organisms/synthetic communities, statistical learning, and dynamical systems modeling in the pursuit of natural design principles for microbial community functions. Whenever possible we anchor ourselves to natural systems through collaborations with field scientists and bioinformatic investigations of ‘omics datasets.
NEWS
- May 2025: We welcome summer REU student Emily Yoon to the lab! Emily is a rising junior at Williams College, and will be working on developing a new model system for studying the community ecology of denitrification.
- April 2025: Undergraduate researcher Aouss Azzouz won another poster award, this time at the Emerging Researchers National Conference in STEM. Also very proud to announce that he will be starting med school at UPenn next Fall. Congrats Aouss!
- February 2025: We are thrilled to have Dominic Cipiti joining the lab as a Microbiology PhD student. He will take a resource-allocation perspective to the study of denitrification physiology.
- January 2025: We welcome Bryce Guidry and Anja Steinert to the lab! Bryce joins as a PhD student through the Biophysics Graduate Program and will be working on single-cell approaches for studying bacteria. Anja will be taking over as lab manager.
- November 2024: Summer REU student Aouss Azzouz won a poster award for presenting his work in the Gowda Lab at ABRCMS! Congrats Aouss!
- July 2024: Our paper on how interactions shape denitrifying communities in the soil microbiome is now out in Nature Microbiology.
- May 2024: Welcome to undergraduates Molly Easton and Aouss Azzouz! Molly joins as an undergraduate lab assistant and Aouss joins as an SROP summer REU student from Earlham College.
- January 2024: The Gowda Lab opens its doors in the Department of Microbiology at the Ohio State University.
PEOPLE
Karna Gowda, PhD [CV]
Principal InvestigatorDepartment of Microbiology
Biophysics Graduate Program
The Ohio State University
Anja Steinert
Lab ManagerBS in Biology, The Ohio State University

Dominic Cipiti
PhD Student, MicrobiologyBS in Biology, Baldwin Wallace University

Bryce Guidry
PhD Student, BiophysicsBS in Biophysics, Emory University
Molly Easton
Undergraduate ResearcherEmily Yoon
Undergraduate Researcher, Williams College
Kuromatsu
DogAlumni
Aouss Azzouz (Earlham College→UPenn Medical School), SROP Undergraduate Researcher
RESEARCH
- The cost of metabolic functionality in bacterial denitrification: Textbooks will tell you that organisms obtain energy via denitrification through a series of reactions starting with nitrate and ending with dinitrogen gas; that is, the process is carried out from start to finish by generalists who do it all. When we look at natural environments, we see that denitrification is very much a community phenomenon, where the pathway is often split up amongst different organisms. The overarching goal of this project is to understand the physiological costs of performing single vs. multiple denitrification reactions, and to use this knowledge to explain and predict the community ecology of denitrifying communities in different environmental contexts.
- Life in the slow lane: Lab studies of microbes typically take place in fast-growth conditions—but organisms in natural environments often grow much more slowly, due to limitations imposed by low nutrient concentrations. The goal of this project to understand physiological and ecological consequences of different environmentally-prevelent forms of nutrient limitation (i.e., P, N, and C limitation) via the development of a high-throughput platform for measuring growth in nutrient-limited conditions.
- Single-cell ‘omics of microbial communities: The fundamental unit of a microbial community is not the population but the single cell—even at the strain-level, there is staggering diversity arising at the genomic and transcriptomic scales, which is essentially papered-over by bulk sequencing measurements. The long term goal of this project is to apply and refine emerging bacterial single cell genomics and transcriptomics approaches (in collaboration with the Sullivan and Prakash labs) to the study of communities, and to develop computational approaches relevant to the unique questions and challenges posed by these measurements in a community context.
PUBLICATIONS
Recent highlights

An old truism in microbial ecology is that "everything is everywhere but the environment selects". But we know that interactions between organisms in microbial communities are widespread—shouldn't that matter as well? In this paper, we show that community assembly is shaped by microbial physiology and interactions, not just environmental factors. Using a statistical approach to analyze global topsoil microbiome data, we uncovered a novel link between denitrification reductase gene abundances and soil pH. Lab experiments and isolate characterizations showed that low pH promotes toxin-antitoxin codependency between strains, explaining statistical patterns observed in soils around the planet.
News & Views by Daniel Amor
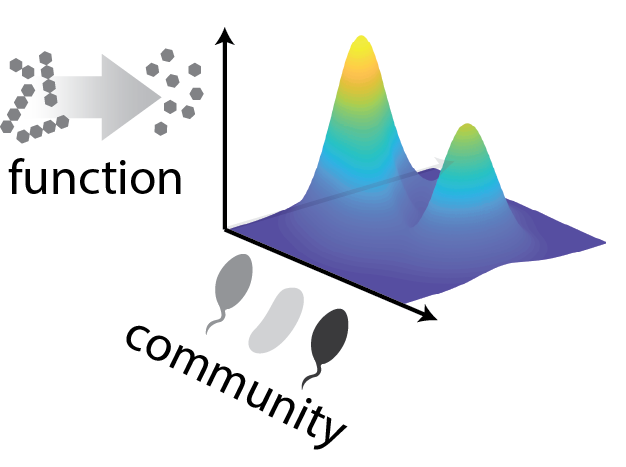
Imagine opening a freezer box full of strains and asking which combination of them would be best at performing a given function (e.g., producing or degrading a compound of interest). Screening all combinations of 10 strains requires at least a thousand experiments, and all combinations of 20 strains requires a million. Here, we show that searching the space of community configurations may in fact be much easier than brute force. We do this by applying statistical learning to the concept of community-function landscapes, showing in datasets and models that these landscapes are often smooth and thus possible to explore with only a handful of experiments.
Dispatch by Avi Flamholz and Dianne Newman
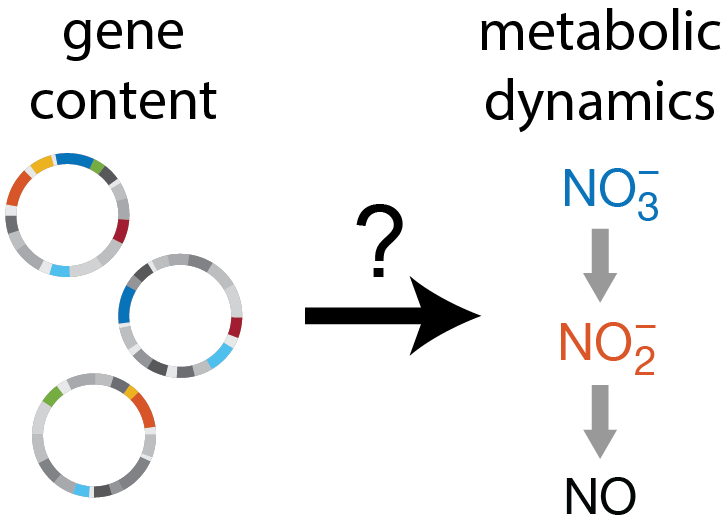
In natural microbial communities, measuring gene content is one of the easiest things to do, while measuring metabolic process rates is one of the hardest. But what if we could predict the latter from the former? In this paper, we establish a proof of concept that this is possible using bacterial denitrification as a model system. Through experimental measurements of synthetic communities composed of diverse isolates, we use statistical learning to map genotypes to phenotypes, and consumer-resource modeling to map phenotypes to community metabolic dynamics.
2024
Crocker, K., Lee, K. K., Chakraverti-Wuerthwein, M., Li, Z., Tikhonov, M., Mani, M., Gowda, K., & Kuehn, S. (2024). Environmentally dependent interactions shape patterns in gene content across natural microbiomes. Nature Microbiology, 9(8), 2022–2037. doi: 10.1038/s41564-024-01752-4
2023
Skwara, A., Gowda, K., Yousef, M., Diaz-Colunga, J., Raman, A. S., Sanchez, A., Tikhonov, M., & Kuehn, S. (2023). Statistically learning the functional landscape of microbial communities. Nature Ecology & Evolution, 7(11), 1823–1833. doi: 10.1038/s41559-023-02197-4
Diaz-Colunga, J., Skwara, A., Gowda, K., Diaz-Uriarte, R., Tikhonov, M., Bajic, D., & Sanchez, A. (2023). Global epistasis on fitness landscapes. Philosophical Transactions of the Royal Society B, 378(1877), 1–20. doi: 10.1098/rstb.2022.0053
2022
Gowda, K., & Kuehn, S. (2022). Microbial biofilms: An ecological tale of Jekyll and Hyde. Current Biology, 32(24), R1349–R1351. doi: 10.1016/j.cub.2022.10.068
Gowda, K., Ping, D., Mani, M., & Kuehn, S. (2022). Genomic structure predicts metabolite dynamics in microbial communities. Cell, 185(3), 530-546.e25. doi: 10.1016/j.cell.2021.12.036
Gopalakrishnappa, C., Gowda, K., Prabhakara, K. H., & Kuehn, S. (2022). An ensemble approach to the structure-function problem in microbial communities. iScience, 25(2), 103761. doi: 10.1016/j.isci.2022.103761
2020
Fraebel, D. T., Gowda, K., Mani, M., & Kuehn, S. (2020). Evolution of Generalists by Phenotypic Plasticity. iScience, 23(11), 1–22. doi: 10.1016/j.isci.2020.101678
PhD
Gandhi, P., Werner, L., Iams, S., Gowda, K., & Silber, M. (2018). A topographic mechanism for arcing of dryland vegetation bands. Journal of the Royal Society Interface, 15(147). doi: 10.1098/rsif.2018.0508
Gowda, K., Iams, S., & Silber, M. (2018). Signatures of human impact on self-organized vegetation in the Horn of Africa. Scientific Reports, 8(1), 1–8. doi: 10.1038/s41598-018-22075-5
Gowda, K., Chen, Y., Iams, S., & Silber, M. (2016). Assessing the robustness of spatial pattern sequences in a dryland vegetation model. Proceedings of the Royal Society A, 472(2187). doi: 10.1098/rspa.2015.0893
Gowda, K., & Kuehn, C. (2015). Early-warning signs for pattern-formation in stochastic partial differential equations. Communications in Nonlinear Science and Numerical Simulation, 22(1–3), 55–69. doi: 10.1016/j.cnsns.2014.09.019
Gowda, K., Riecke, H., & Silber, M. (2014). Transitions between patterned states in vegetation models for semiarid ecosystems. Physical Review E, 89(2), 1–8. doi: 10.1103/PhysRevE.89.022701